CAG Center for Endotheliomics (ENDO)
Center for Endotheliomics
The Center for Endotheliomics’ ambition is that research in Eastern Denmark lead to breakthroughs in the treatment of critically ill patients in need of intensive care patient who enter hospitals with either trauma, cardiac arrest or severe sepsis. For this patient group, survival is counted in hours, and up to half of the patients die within 72 hours – often as a result of organ failure.
Organ failure – or multi-organ failure – is when the organs stop functioning, which happens to the most critically ill patients in intensive care. However, whether the patient develops organ failure is largely determined by the endothelium, which is the cell layer that lines the inside of all blood vessels. How the individual patient’s endothelium reacts to shock, trauma, sepsis or cardiac arrest determines how severe organ failure the patient will develop – and thus the patient’s risk of death.
Mathematical model reconstructs the cell’s metabolism
The CAG brings together clinical experts in trauma, sepsis and cardiac arrest with experts in systems biology, biotechnology and bioinformatics. Together we will generate new knowledge about critical illness and multi-organ failure.
We have developed a method based on mathematical computer models that reconstructs the metabolism of the endothelial cell and thus its function (phenotype).
These models use metabolites in the patient’s blood plasma as well as genetic variations in the patient’s DNA to predict the condition of the endothelial cell. In this way, we can better understand how the individual patient’s endothelial cells respond to acute critical illness with shock.
The aim of the CAG is to exploit the new knowledge about the importance of endothelial cells for multi-organ failure and with mathematical modeling and systems biology understand the difference between the endothelial response in acute critical illness in surviving patients and in those who die. When we succeed, we can intervene and adjust the endothelium in time before the patient develops organ failure.
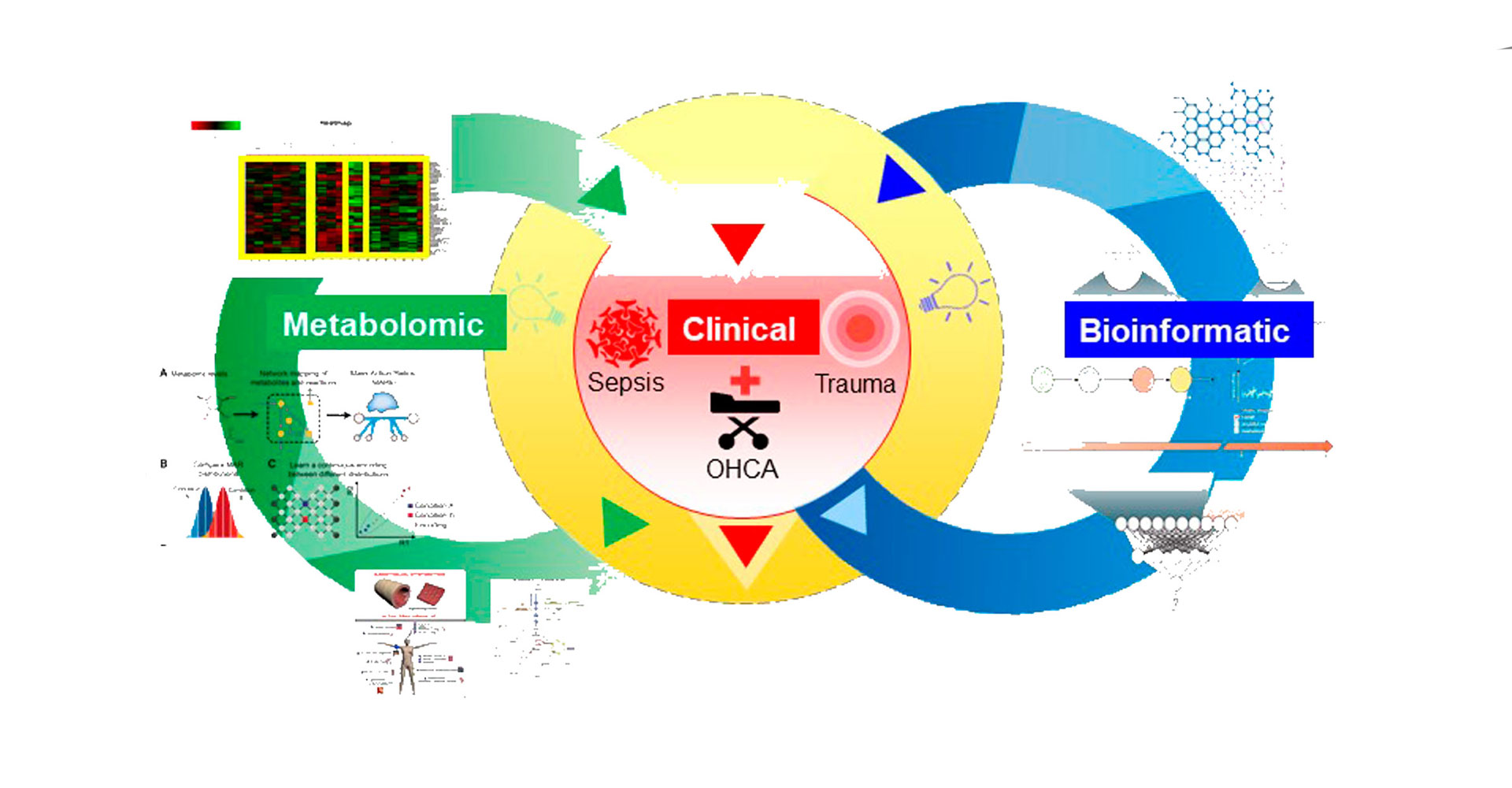

Center for Endotheliomics introduces a novel research field focusing on the microvascular endothelium at the systemic level and that the individual patient’s cellular response (ENDOTYPES) to critical illness is pre-determined and differ between patients also with the same disease explaining the observed differences in outcome.
Center for Endotheliomics aim to improve our understanding of how the patients ENDOTYPE affects development of multiorgan dysfunction syndrome (MODS) when challenged with acute critical illness and to translate this novel knowledge in to improved diagnostic and therapeutic capabilities.
Center for Endotheliomics introduces genome scale metabolic models as the scaffold for multi-omics data integration and computational modeling as tools to decipher the pathophysiology responsible for MODS development and progression clinically. Additionally, supervised machine learning algorithms will be applied to data from electronic patient records, Danish registries and clinical databases of these critically ill patients to characterize the clinical disease trajectories resulting from the individual patients ENDOTYPE, thereby, laying the foundation for novel patient stratifications and therapeutic interventions.
Our research projects
REDEEM
Using metabolic system analysis, REDEEM examines the significance of endotheliopathies for disease development and survival in patients with pulmonary arterial hypertension.
The study is a collaboration between the Department of Clinical Immunology and the Heart Department at Rigshospitalet.
The study is based on patient samples and clinical data collected in the DAN-PH register.
REDEEM is supported by the Novo Nordisk Foundation.
COMBAT-TOX

COMBAT-TOX is a randomized, double-blinded, multicenter study investigating the effect of infusion of selective beta-blockers vs. placebo (saline) in 200 patients with septic shock. The primary endpoint is 30-day mortality.
The study is coordinated by the CAG unit for clinical studies in collaboration with the intensive care units at Nordsjællands Hospital, Bispebjerg Hospital and Herlev Hospital.
Patient inclusion has not started yet.
COMBAT-TOX is supported by the Innovationsfonden.
COMBAT ARF

COMBAT-ARF is a randomized, double-blinded, clinical trial investigating the effect of continuous infusion of prostacyclin (1 ng/kg/min) vs. placebo (saline) for 72 hours on the number of days outside the intensive care unit in 400 patients with acute lung failure. The primary endpoint is 28-day mortality.
The study is coordinated by the CAG unit for clinical studies at Rigshospitalet in collaboration with the intensive care units Nordsjællands Hospital, Bispebjerg Hospital, Herlev Hospital and Zealand University Hospital Køge
Patient inclusion is ongoing.
COMBAT-ARF is supported by the Novo Nordisk Foundation and the Alfred Benzon Foundation.
PRIORITY

PRIORITY is a randomized, single-center study investigating the effect of infusion with blood, screened for durability, in 3,000 patients in need of blood products.
The study is coordinated by the CAG unit for clinical studies at Rigshospitalet. The blood bank is one department that ensures blood supply at all hospitals in the Capital Region.
Patient inclusion has not started yet.
PRIORITY is supported by the Innovation Fund.
ENDO-OMICS

The ENDO-OMICS is a multicenter, prospective observational exploratory study in 1.000 patients admitted to the intensive care unit (ICU) 4131 at Rigshospitalet, Bispebjerg Hospital, Hvidovre Hospital, Køge Hospital and at the ICU at Nordsjællands Hospital.
Blood samples will be analyzed by genomic, transcriptomic, proteomic and metabolomic techniques with the overall aim to develop computational modeling of the endothelial cell to deepen the biological understanding/ paving the way for more precise diagnostics and new treatments.
Patient recruiting completed.
SHINE-TRAUMA

SHINE-TRAUMA is a randomized, double-blind, clinical trial investigating the effect of continuous infusion of prostacyclin (1 ng/kg/min) vs. placebo (saline) for 72 hours on number of ICU free days in 220 trauma patients with haemorrhagic shock.
The study is coordinated by the CAG Trial Unit at Rigshospitalet and clinical partners are the trauma centers at the Rigshospitalet and the University Hospitals in Odense, Århus and Oslo.
Patient recruiting completed.
SHINE-TRAUMA is funded by Novo Nordisk Foundation and Danske Regioners Medicinpulje.
Hillerød Metabolomics
 Hillerød Metabolomics is a prospective clinical study investigating metabolic changes in whole blood in patients with acute critical illness.
Hillerød Metabolomics is a prospective clinical study investigating metabolic changes in whole blood in patients with acute critical illness.
The purpose is to measure the concentrations of neurotransmitters (metabolites) in plasma from patients with acute critical illness and in healthy controls, and to use the results to provide a better understanding of the significance of these neurotransmitters for endothelial cell damage.
The partners are Intensive Care Unit (ITA), Hillerød Hospital and Rigshospitalet.
Patient recruiting completed.
CAG Center for Endotheliomics has collaborators nationally and around the world.
National collaborators
COMBAT-SHINE
- Peter Søe-Jensen, MD, PhD, EDIC, Department of Intensive Care, Herlev Hospital
- Klaus Tjelle Kristiansen, MD, Department of Intensive Care, Hvidovre Hospital
University of Copenhagen
- Theis Lange, Section of Biostatistics, University of Copenhagen
- Prof. Karsten Vrangbæk, Department of Political Science and Public Health, University of Copenhagen
International collaborators
TACTIC/INTRN
- Prof. Karim Brohi, Centre for Trauma Sciences, Queen Mary University of London, UK
- Prof. Simon Stanworth, Radcliff Department of Medicine, University of Oxford, UK
- Prof. Nicole Juffermans, Department of Intensive Care Medicine, Academic MedicalCenter, Amsterdam, The Netherlands
- Prof. Marc Maegele, Department of Traumatology and Orthopedic Surgery Cologne-Merheim Medical Center (CMMC), Germany
- Christina Gaarder, MD, PhD, Department of Traumatology, Oslo University Hospital, Norway
Center for Translational Injury Research (CeTIR)
- Prof. Charles E. Wade PhD, Department of Surgery, McGovern Medical School, UTHealth, Houston, USA
- Assistant Prof. Jessica C. Cardenas, Ph.D, Department of Surgery, McGovern Medical School, UTHealth, Houston, USA
Center for Systems Biology, Iceland University
- Prof. Ottar Rolfsson, MSc, PhD, Center for Systems Biology, University of Iceland, Iceland
- Adrian Lopez Garcia de Lomana, PhD, Center for Systems Biology, University of Iceland, Iceland
Harvard Medical School, Boston, USA
- Prof. Joseph Loscalzo, MD, PhD, MA, Head of Department of Medicine, Brigham and Women´s Hospital, Harvard Medical School, Boston, USA
CAG Chairs
-
Pär Ingemar Johansson Professor, Senior Consultant, Department of Clinical Immunology, Rigshospitalet, The Capital Region of Denmark
-
Bernhard Palsson Professor, CEO, Novo Nordisk Foundation Center for Biosustainability, Technical University of Denmark - DTU
CAG Junior Chairs
CAG Key Members
-
Lars K. Nielsen Professor, CSO, Novo Nordisk Foundation Center for Sustainability Technical University of Copenhagen
-
Christian Igel Professor, dr. habil., Department of Computer Science, Faculty of Science University of Copenhagen
-
-
Anders Perner Professor, senior consultant, Intensive Care, Rigshospitalet The Capital Region of Denmark
-
Christian Hassager Professor, senior consultant, Department of Cardiology, rigshospitalet The Capital Region of Copenhagen
-
Morten Bestle Professor, senior consultant, Intensive Care, Nordsjaellands Hospital, Hilleroed The Capital Region of Denmark
-
Jesper Kjærgaard Senior consultant, Department of Cardiology, Rigshospitalet The Capital Region of Denmark
-
Jakob Stensballe Senior consultant, Trauma Anaesthesia, Rigshospitalet The Capital Region of Denmark
-
-
 Jørn Carlsen Associate professor, Senior consultant, Department of cardiology, Rigshospitalet the capital region of Copenhagen
Jørn Carlsen Associate professor, Senior consultant, Department of cardiology, Rigshospitalet the capital region of Copenhagen -
 Ole Mathiesen PROFESSOR, SENIOR CONSULTANT, DEPARTMENT OF ANAESTHESIA, ZEALAND UNIVERSITY HOSPITAL, KØGE, REGION ZEALAND
Ole Mathiesen PROFESSOR, SENIOR CONSULTANT, DEPARTMENT OF ANAESTHESIA, ZEALAND UNIVERSITY HOSPITAL, KØGE, REGION ZEALAND -
Morten Juhl Head of in vitro stem cell lab, Cardiology stem cell centre, Rigshospitalet, The Capital Region of Denmark
-
Mads Nielsen Professor, Department of Computer Science, Faculty of Science, University of Copenhagen
Science
-
-

-
 Nathan Weinstein PHD, SENIOR RESEARCHER
Nathan Weinstein PHD, SENIOR RESEARCHER -
-
-
-
-
-
-
-
-
-
Funding and strategy
Laboratory Technologists
-
 Randa Zo El-Ghina B.SC., HEAD OF CLINICAL LABORATORY
Randa Zo El-Ghina B.SC., HEAD OF CLINICAL LABORATORY -
Clinical trials
-

-
-

-
 Tenna Schnack BSCN, STUDY NURSE
Tenna Schnack BSCN, STUDY NURSE
Publications
Johansson PI, Henriksen HH, Karvelsson ST, Rolfsson Ó, Schønemann-Lund M, Bestle MH, McGarrity S.Eur J Med Res. 2024 Jan 20;29(1):71. doi: 10.1186/s40001-023-01612-7.PMID: 38245777
Horan DE, Kielsen K, Weischendorff SW, Sørum ME, Kammersgaard MB, Ifversen M, Nielsen C, Ryder LP, Johansson PI, Müller K.Transpl Immunol. 2024 Feb;82:101975. doi: 10.1016/j.trim.2023.101975. Epub 2023 Dec 19.PMID: 38122992
Dujardin RWG, Kleinveld DJB, van den Brom CE, Geeraedts LMG Jr, Beijer E, Gaarder C, Brohi K, Stanworth S, Johansson PI, Stensballe J, Maegele M, Juffermans NP.J Trauma Acute Care Surg. 2024 May 1;96(5):831-837. doi: 10.1097/TA.0000000000004235. Epub 2023 Dec 8.PMID: 38079234
Prostacyclin in trauma patients with hemorrhagic shock: A randomized clinical trial.
Johansson PI, Fenger Eriksen C, Bovbjerg PE, Gaarder C, Pall M, Henriksen HH, Pedersen KH, Vigstedt M, Lange T, Næss PA, Strømgaard Andersen M, Kirkegaard H, Stensballe J.J Trauma Acute Care Surg. 2024 Mar 1;96(3):476-481. doi: 10.1097/TA.0000000000004150. Epub 2023 Nov 14.PMID: 37962189 Clinical Trial.
Lipidomic signatures align with inflammatory patterns and outcomes in critical illness.
Wu J, Cyr A, Gruen DS, Lovelace TC, Benos PV, Das J, Kar UK, Chen T, Guyette FX, Yazer MH, Daley BJ, Miller RS, Harbrecht BG, Claridge JA, Phelan HA, Zuckerbraun BS, Neal MD, Johansson PI, Stensballe J, Namas RA, Vodovotz Y, Sperry JL, Billiar TR; PAMPer study group. Nat Commun. 2022 Nov 10;13(1):6789. doi: 10.1038/s41467-022-34420-4.PMID: 36357394
High-dimensional proteomics identifies organ injury patterns associated with outcomes in human trauma.
Li SR, Moheimani H, Herzig B, Kail M, Krishnamoorthi N, Wu J, Abdelhamid S, Scioscia J, Sung E, Rosengart A, Bonaroti J, Johansson PI, Stensballe J, Neal MD, Das J, Kar U, Sperry J, Billiar TR.J Trauma Acute Care Surg. 2023 Jun 1;94(6):803-813. doi: 10.1097/TA.0000000000003880. Epub 2023 Feb 13.PMID: 36787435 Review.
Shock-Driven Endotheliopathy in Trauma Patients Is Associated with Leucocyte Derived Extracellular Vesicles.
Dujardin RWG, Kisters JEC, Wirtz MR, Hajji N, Tuip-de Boer AM, Stensballe J, Johansson PI, Brohi K, Davenport RA, Gaarder C, Stanworth S, Maegele M, Nieuwland R, van der Pol E, Juffermans NP.Int J Mol Sci. 2022 Dec 15;23(24):15990. doi: 10.3390/ijms232415990.PMID: 36555630
Impact of high-dose glucocorticoid on endothelial damage after liver resection – a double-blinded randomized substudy.
Pitter SELT, Steinthorsdottir KJ, Johansson PI, Nørgaard P, Schultz N, Kehlet H, Aasvang EK.Eur J Gastroenterol Hepatol. 2022 Nov 1;34(11):1178-1186. doi: 10.1097/MEG.0000000000002449. Epub 2022 Sep 19.PMID: 36170688 Clinical Trial.
Impact of high-dose glucocorticoid on endothelial damage after liver resection – a double-blinded randomized substudy.
Pitter SELT, Steinthorsdottir KJ, Johansson PI, Nørgaard P, Schultz N, Kehlet H, Aasvang EK.Eur J Gastroenterol Hepatol. 2022 Sep 20. doi: 10.1097/MxhEG.0000000000002449. Online ahead of print.PMID: 36165063
Endothelial Cell Phenotypes Demonstrate Different Metabolic Patterns and Predict Mortality in Trauma Patients.
Henriksen HH, Marín de Mas I, Nielsen LK, Krocker J, Stensballe J, Karvelsson ST, Secher NH, Rolfsson Ó, Wade CE, Johansson PI.Int J Mol Sci. 2023 Jan 23;24(3):2257. doi: 10.3390/ijms24032257.PMID: 36768579
The Effect of Targeted Temperature Management on the Metabolome Following Out-of-Hospital Cardiac Arrest.
Beske RP, Obling LER, Bro-Jeppesen J, Nielsen N, Meyer MAS, Kjaergaard J, Johansson PI, Hassager C.Ther Hypothermia Temp Manag. 2023 May 23. doi: 10.1089/ther.2022.0065. Online ahead of print.PMID: 37219970
A Protocol for the Automatic Construction of Highly Curated Genome-Scale Models of Human Metabolism.
Marin de Mas I, Herand H, Carrasco J, Nielsen LK, Johansson PI.Bioengineering (Basel). 2023 May 10;10(5):576. doi: 10.3390/bioengineering10050576.PMID: 37237646
Major open abdominal surgery is associated with increased levels of endothelial damage and interleukin-6.
Gregersen JS, Bazancir LA, Johansson PI, Sørensen H, Achiam MP, Olsen AA.Microvasc Res. 2023 Jul;148:104543. doi: 10.1016/j.mvr.2023.104543. Epub 2023 May 6.PMID: 37156371
Novel subgroups in acute respiratory failure based on the trajectories of three endotheliopathy biomarkers: A cohort study.
Schønemann-Lund M, Itenov TS, Larsson JE, Lindegaard B, Johansson PI, Bestle MH.Acta Anaesthesiol Scand. 2023 Apr 12. doi: 10.1111/aas.14246. Online ahead of print.PMID: 37042167
Prehospital tranexamic acid is associated with a dose-dependent decrease in syndecan-1 after trauma: A secondary analysis of a prospective randomized trial.
Gruen DS, Brown JB, Guyette FX, Johansson PI, Stensballe J, Li SR, Leeper CM, Eastridge BJ, Nirula R, Vercruysse GA, O’Keeffe T, Joseph B, Neal MD, Sperry JL.J Trauma Acute Care Surg. 2023 May 1. doi: 10.1097/TA.0000000000003955. Online ahead of print.PMID: 37125811
Metabolic systems analysis identifies a novel mechanism contributing to shock in patients with endotheliopathy of trauma (EoT) involving thromboxane A2 and LTC 4
Hanne H Henriksen, Igor Marín de Mas, Helena Herand, Joseph Krocker, Charles E Wade, Pär I Johansson. Matrix Biol Plus. 2022 Jun 18;15:100115. doi: 10.1016/j.mbplus.2022.100115. eCollection 2022 Aug. PMID: 35813244
Exploratory Investigation of the Plasma Proteome Associated with the Endotheliopathy of Trauma
Joseph D. Krocker, Kyung Hyun Lee, Hanne H. Henriksen, Yao-Wei Willa Wang, Erwin M. Schoof, Sigurdur T. Karvelsson, Óttar Rolfsson, Pär I. Johansson, Claudia Pedroza, Charles E. Wade Int. J. Mol. Sci. 2022, 23(11), 6213; https://doi.org/10.3390/ijms23116213
Intervening on the storage time of RBC units and its effects on adverse recipientoutcomes using real-world data
Peter Bruun-Rasmussen, Per Kragh Andersen, Karina Banasik, Søren Brunak, Pär Ingemar Johansson Blood (2022) 139 (25): 3647–3654. https://doi.org/10.1182/blood.2022015892
Targeted plasma metabolomics in resuscitated comatose out-of-hospital cardiac arrest patients
Rasmus Paulin Beske, Hanne H Henriksen, Laust Obling, Jesper Kjærgaard, John Bro-Jeppesen, Niklas Nielsen, Pär I Johanson, Christian Hassager. Resuscitation. 2022 Jun 23; S0300-9572(22)00573-1. doi: 10.1016/j.resuscitation. 2022.06.010.
An explorative metabolomic analysis of the endothelium in pulmonary hypertension.
Carlsen J, Henriksen HH, Marin de Mas I, Johansson PI.Sci Rep. 2022 Aug 2;12(1):13284. doi: 10.1038/s41598-022-17374-x.PMID: 35918401
Estimating the effect of donor sex on red blood cell transfused patient mortality: A retrospective cohort study using a targeted learning and emulated trials-based approach
Bruun-Rasmussen P, Andersen PK, Banasik K, Brunak S, Johansson PI. eClinicalMedicinePublished: August 27, 2022
Evaluation of the systemic inflammatory response, endothelial cell dysfunction, and postoperative morbidity in patients, receiving perioperative corticosteroid, developing severe mesenteric traction syndrome – an exploratory study.
Olsen AA, Strandby RB, Johansson PI, Sørensen H, Svendsen LB, Achiam MP.Langenbecks Arch Surg. 2022 Apr 9. doi: 10.1007/s00423-022-02507-7. Online ahead of print.PMID: 35397681
Metabolic Response in Endothelial Cells to Catecholamine Stimulation Associated with Increased Vascular Permeability.
López García de Lomana A, Vilhjálmsson AI, McGarrity S, Sigurðardóttir R, Anuforo Ó, Viktorsdóttir AR, Kotronoulas A, Bergmann A, Franzson L, Halldórsson H, Henriksen HH, Wade CE, Johansson PI, Rolfsson Ó.Int J Mol Sci. 2022 Mar 15;23(6):3162. doi: 10.3390/ijms23063162.PMID: 35328583 Free PMC article.
The effect of prostacyclin infusion on markers of endothelial activation and damage in mechanically ventilated patients with SARS-CoV-2 infection.
Vigstedt M, Søe-Jensen P, Bestle MH, Clausen NE, Kristiansen KT, Lange T, Stensballe J, Perner A, Johansson PI.J Crit Care. 2022 Feb 17;69:154010. doi: 10.1016/j.jcrc.2022.154010. Online ahead of print.PMID: 35183892 Free PMC article.
Endotheliopathy is associated with slower liberation from mechanical ventilation: a cohort study.
Schønemann-Lund M, Itenov TS, Larsson JE, Lindegaard B, Johansson PI, Bestle MH.Crit Care. 2022 Jan 30;26(1):33. doi: 10.1186/s13054-021-03877-y.PMID: 35094711 Free PMC article.
Multi-omic analysis in injured humans: Patterns align with outcomes and treatment responses.
Wu J, Vodovotz Y, Abdelhamid S, Guyette FX, Yaffe MB, Gruen DS, Cyr A, Okonkwo DO, Kar UK, Krishnamoorthi N, Voinchet RG, Billiar IM, Yazer MH, Namas RA, Daley BJ, Miller RS, Harbrecht BG, Claridge JA, Phelan HA, Zuckerbraun BS, Johansson PI, Stensballe J, Morrissey JH, Tracy RP, Wisniewski SR, Neal MD, Sperry JL, Billiar TR; PAMPer study group.Cell Rep Med. 2021 Dec 21;2(12):100478. doi: 10.1016/j.xcrm.2021.100478. eCollection 2021 Dec 21.PMID: 35028617 Free PMC article.
Shock-Induced Endothelial Dysfunction is Present in Patients With Occult Hypoperfusion After Trauma.
Kregel HR, Hatton GE, Isbell KD, Henriksen HH, Stensballe J, Johansson PI, Kao LS, Wade CE.Shock. 2022 Jan 1;57(1):106-112. doi: 10.1097/SHK.0000000000001866.PMID: 34905531
Prostacyclin in Intubated Patients with COVID-19 and Severe Endotheliopathy: A Multicenter, Randomized Clinical Trial.
Johansson PI, Søe-Jensen P, Bestle MH, Clausen NE, Kristiansen KT, Lange T, Stensballe J, Perner A.Am J Respir Crit Care Med. 2022 Feb 1;205(3):324-329. doi: 10.1164/rccm.202108-1855OC.PMID: 34813414 Free PMC article. Clinical Trial.
Endothelial dysfunction and thromboembolism in children, adolescents, and young adults with acute lymphoblastic leukemia.
Andrés-Jensen L, Grell K, Rank CU, Albertsen BK, Tuckuviene R, Linnemann Nielsen R, Lynggaard LS, Jarvis KB, Quist-Paulsen P, Trakymiene SS, Semaškevičienė R, Saks K, Jonsson OG, Frandsen TL, Johansson PI, Schmiegelow K.Leukemia. 2022 Feb;36(2):361-369. doi: 10.1038/s41375-021-01383-2. Epub 2021 Aug 13.PMID: 34389803
Severe mesenteric traction syndrome is associated with increased systemic inflammatory response, endothelial dysfunction, and major postoperative morbidity.
Olsen AA, Strandby RB, Nerup N, Johansson PI, Svendsen LB, Achiam MP.Langenbecks Arch Surg. 2021 Nov;406(7):2457-2467. doi: 10.1007/s00423-021-02111-1. Epub 2021 Mar 8.PMID: 33686490
Efficacy and safety of iloprost in trauma patients with haemorrhagic shock-induced endotheliopathy-Protocol for the multicentre randomized, placebo-controlled, blinded, investigator-initiated shine-trauma trial.
Johansson PI, Eriksen CF, Schmal H, Gaarder C, Pall M, Henriksen HH, Bovbjerg P, Lange T, Naess PA, Nielsen C, Kirkegaard H, Stensballe J.Acta Anaesthesiol Scand. 2021 Jan 3;65(4):551-7. doi: 10.1111/aas.13776. Online ahead of print.PMID: 33393084 Free PMC article.
The effect of prostacyclin (Iloprost) infusion at a dose of 1 ng/kg/min for 72 hours compared to placebo in mechanically ventilated patients with COVID-19: A structured summary of a study protocol for a randomized controlled trial.
Johansson PI, Bestle M, Søe-Jensen P, Kristiansen KT, Stensballe J, Clausen NE, Perner A.Trials. 2020 Aug 26;21(1):746. doi: 10.1186/s13063-020-04696-2.PMID: 32847626 Free PMC article. Clinical Trial.
Endothelial Dysfunction is Associated With Increased Incidence, Worsened Severity, and Prolonged Duration of Acute Kidney Injury After Severe Trauma.
Hatton GE, Isbell KD, Henriksen HH, Stensballe J, Brummerstedt M, Johansson PI, Kao LS, Wade CE.Shock. 2021 Mar 1;55(3):311-315. doi: 10.1097/SHK.0000000000001638.PMID: 32826819 Free PMC article.
A randomised double-blind pilot trial comparing a mean arterial pressure target of 65 mm Hg versus 72 mm Hg after out-of-hospital cardiac arrest.
Grand J, Meyer AS, Kjaergaard J, Wiberg S, Thomsen JH, Frydland M, Ostrowski SR, Johansson PI, Hassager C.Eur Heart J Acute Cardiovasc Care. 2020 Nov;9(4_suppl):S100-S109. doi: 10.1177/2048872619900095. Epub 2020 Jan 31.PMID: 32004081 Clinical Trial.
Efficacy and safety of iloprost in patients with septic shock-induced endotheliopathy-Protocol for the multicenter randomized, placebo-controlled, blinded, investigator-initiated trial.
Bestle MH, Clausen NE, Søe-Jensen P, Kristiansen KT, Lange T, Johansson PI, Stensballe J, Perner A.Acta Anaesthesiol Scand. 2020 May;64(5):705-711. doi: 10.1111/aas.13546. Epub 2020 Feb 3.PMID: 31950481 Free PMC article. Clinical Trial.
Low dose Iloprost effect on platelet aggregation in comatose out-of-hospital cardiac arrest patients: A predefined sub-study of the ENDO-RCA randomized -phase 2- trial.
Meyer ASP, Ostrowski SR, Kjærgaard J, Frydland M, Thomsen JH, Johansson PI, Hassager C.J Crit Care. 2020 Apr;56:197-202. doi: 10.1016/j.jcrc.2019.12.025. Epub 2019 Dec 30.PMID: 31945586 Clinical Trial.
Endothelial glycocalyx shedding in patients with burns.
Welling H, Henriksen HH, Gonzalez-Rodriguez ER, Stensballe J, Huzar TF, Johansson PI, Wade CE.Burns. 2020 Mar;46(2):386-393. doi: 10.1016/j.burns.2019.05.009. Epub 2019 Dec 20.PMID: 31866179
“Endothelial Dysfunction in Resuscitated Cardiac Arrest (ENDO-RCA): Safety and efficacy of low-dose Iloprost, a prostacyclin analogue, in addition to standard therapy, as compared to standard therapy alone, in post-cardiac-arrest-syndrome patients.”.
Meyer ASP, Johansson PI, Kjaergaard J, Frydland M, Meyer MAS, Henriksen HH, Thomsen JH, Wiberg SC, Hassager C, Ostrowski SR.Am Heart J. 2020 Jan;219:9-20. doi: 10.1016/j.ahj.2019.10.002. Epub 2019 Oct 21.PMID: 31710844 Clinical Trial.
Co-administration of iloprost and eptifibatide in septic shock (CO-ILEPSS)-a randomised, controlled, double-blind investigator-initiated trial investigating safety and efficacy.
Berthelsen RE, Ostrowski SR, Bestle MH, Johansson PI.Crit Care. 2019 Sep 5;23(1):301. doi: 10.1186/s13054-019-2573-8.PMID: 31488213 Free PMC article. Clinical Trial.
Metabolic Systems Analysis of Shock-Induced Endotheliopathy (SHINE) in Trauma: A New Research Paradigm.
Henriksen HH, McGarrity S, SigurÐardóttir RS, Nemkov T, D’Alessandro A, Palsson BO, Stensballe J, Wade CE, Rolfsson Ó, Johansson PI.Ann Surg. 2020 Dec;272(6):1140-1148. doi: 10.1097/SLA.0000000000003307.PMID: 31274658
If your inquiry concerns your symptoms, medical history and treatment, please contact your private doctor.







